NYMedic828
Forum Deputy Chief
- 2,094
- 3
- 36
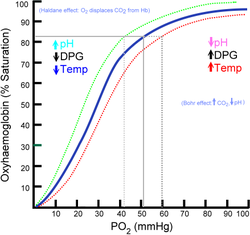
I am having a little trouble comprehending the Bohr effect and can't really find any good articles/texts on it outside of wikipedia.
Is it basically just saying that when the acid content of cells in working tissues becomes higher, it allows for easier exchange of CO2 with oxyhemoglobin? Or am i completely on the wrong path?
My interest basically stemmed from reading up on hyperventilation and effects of respiratory alkalosis.
Is it basically just saying that when the acid content of cells in working tissues becomes higher, it allows for easier exchange of CO2 with oxyhemoglobin? Or am i completely on the wrong path?
My interest basically stemmed from reading up on hyperventilation and effects of respiratory alkalosis.
Last edited by a moderator: