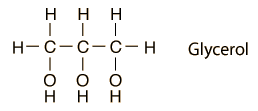In regard to assessment and treatment, I don't feel like science helps a lot. I kinda feel like knowing the science behind medicine are just fun facts.
Understanding chemical nomenclature is fun such as
1 = Meth-
2 = Eth-
3 = Prop-
4 = But-
5 = Pent-
6 = Hex-
7 = Hept-
8 = Oct-
9 = Non-
10 = Dec-
An alkane is carbons (C) fully saturated with hydrogrens (H). C(x)H(x+2)
Image taken from
here.
Methane (CH4), ONE carbon (meth-)
Ethane (C2H6), TWO carbons (eth-)
Propane (C3H8), THREE carbons (prop-)
Butane (C4H10), FOUR carbons (but-)
Pent-
Hex-
Hept-
Oct-
Non-
Dec-
-ol is an alcohol. Replace
something with a hydroxyl group (-OH).
Ethane is C2H6. Ethanol (EtOH) is C2H6O or C2H5OH. Replace a solo hydrogen with a hydroxyl.
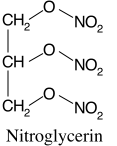
Nitroglycerin (NTG in the United States) = Glyceryl Trinitrate (GTN outside of the United States).
Glycerol = Propanol-1,2,3
Recall that Propane is C3H8. Propanol-1,2,3 is replace the 1st, 2nd, and 3rd solo hydrogens with hydroxyl. That would make it C3H8O3.
Image taken from
here.
Tri- is also three. Nitrate (NO3) is a nitrogen with three oxygen atoms. For glyceryl trinitrate, replace the three hydroxyls (-OH) with nitrates (NO3). That would make it C3H5N3O9.
Image taken from
here. Found via
Google.
I personally visualize nitroglycerin the way it is on
Wikipedia, but I felt that would be more confusing for you guys. They don't draw lines for the hydrogens and the points/bends are carbons.
Chemical nomenclature is also useful for remembering the catecholamines: dopamine, norepinephrine, and epinephrine.
Catechol = C6H4OHOH
Image taken from
here.
-amine = NH2
So a catecholamine is probably gonna have catechol and an amine. Remember that dopamine, norepinephrine, and epinephrine are catecholamines.
Dopamine is the most basic.
Image taken from
Wikipedia.
Note: It is not drawing out the hydogens (H), but they are there. The carbons are where the point/bends are.
Norepinephrine is the second most basic. Just add a hydroxyl (-OH) to dopamine. Nor- for normal.
Image taken from
Wikipedia.
Epinephrine is the most complex. Add a methyl (CH3) to norepinephrine.
Image taken from
Wikipedia.
You can actually apply this to a lot of stuff, but I don't find it particularly useful more than figuring out what kinda of tattoos people have.
Although I learned the concept of diffusion (going from an area of high concentration to low concentration, active versus passive transport) and pressures in high school chemistry (the highest level chemistry I have learned, I have not taken a college level course), I was pretty mind blown how important these were for our body eg the exchange of oxygen and carbon dioxide at the aveoli, movement of ions in the depolarization-repolarization cycle.
I dunno... I don't feel like much of it is useful. They are just fun facts. I feel like I get more bang for my bucks outta learning algorithms, but use some sound judgement when applying those algorithms eg ACLS tachycardia algorithm & sepsis. Don't cardiovert somebody who is tachycardic and in septic shock, lol.